MOVES® SLC™
Put Tomorrow’s Lifesaving Capabilities in Your Hands. Today.
Military, humanitarian and emergency medical units are modernizing for large scale combat operations (LSCO), multi-domain operations (MDO), disaster relief, mass casualty and other emergency scenarios.
The Challenge
These environments can be austere, hard to reach, overwhelmed by the volume of casualties, challenged by extended hold times in ill equipped places of opportunity, isolated by interrupted supply chain, facing the tyranny of distance when evacuating in vehicles of opportunity – in any combination.
Meeting the Challenge
Medical units need to be agile, mobile and self-sufficient. And they must be supported by technology that is smaller, lighter, more durable, intuitive, easy to maintain that brings critical care capabilities as close to the centre of need as possible.
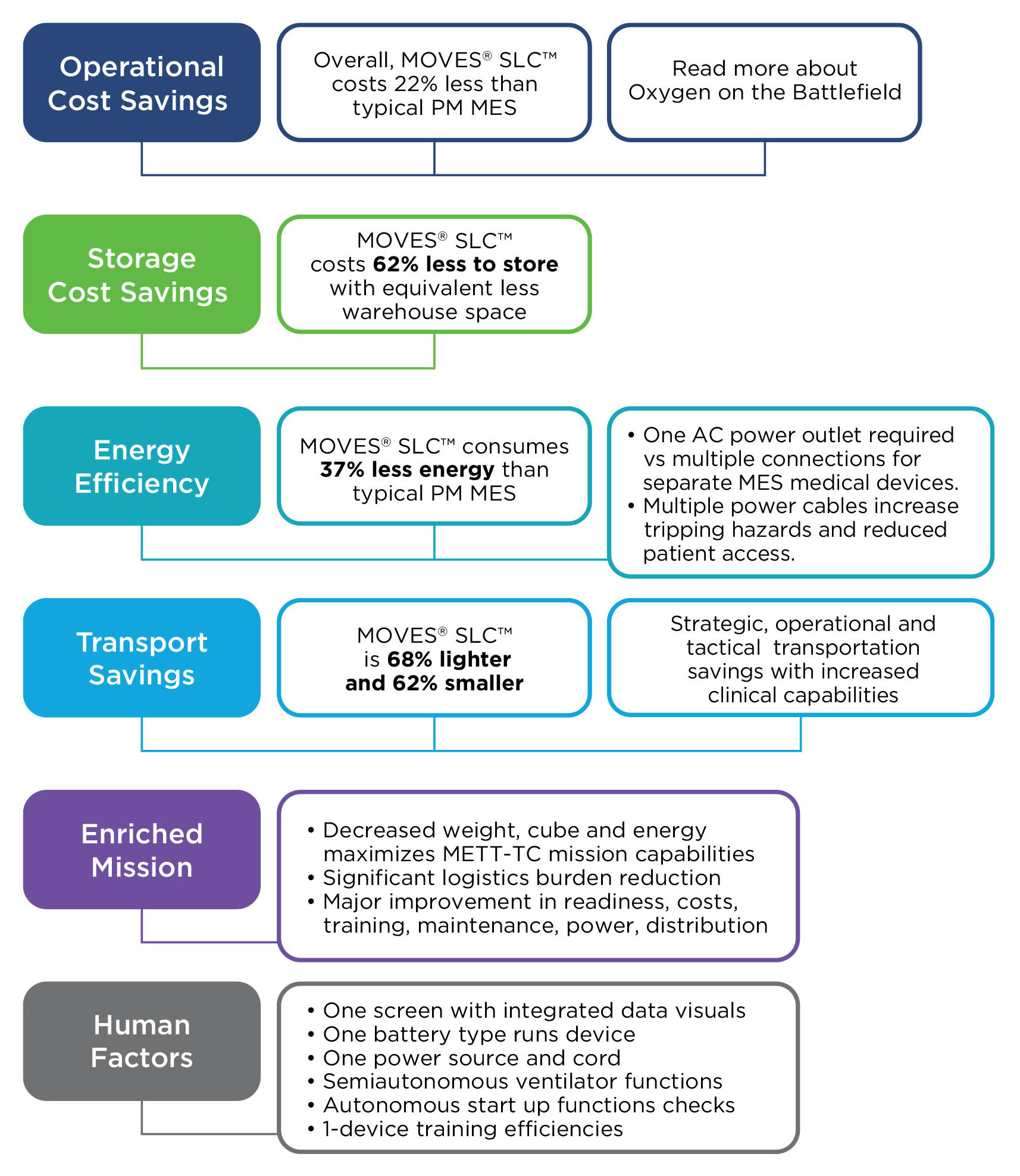
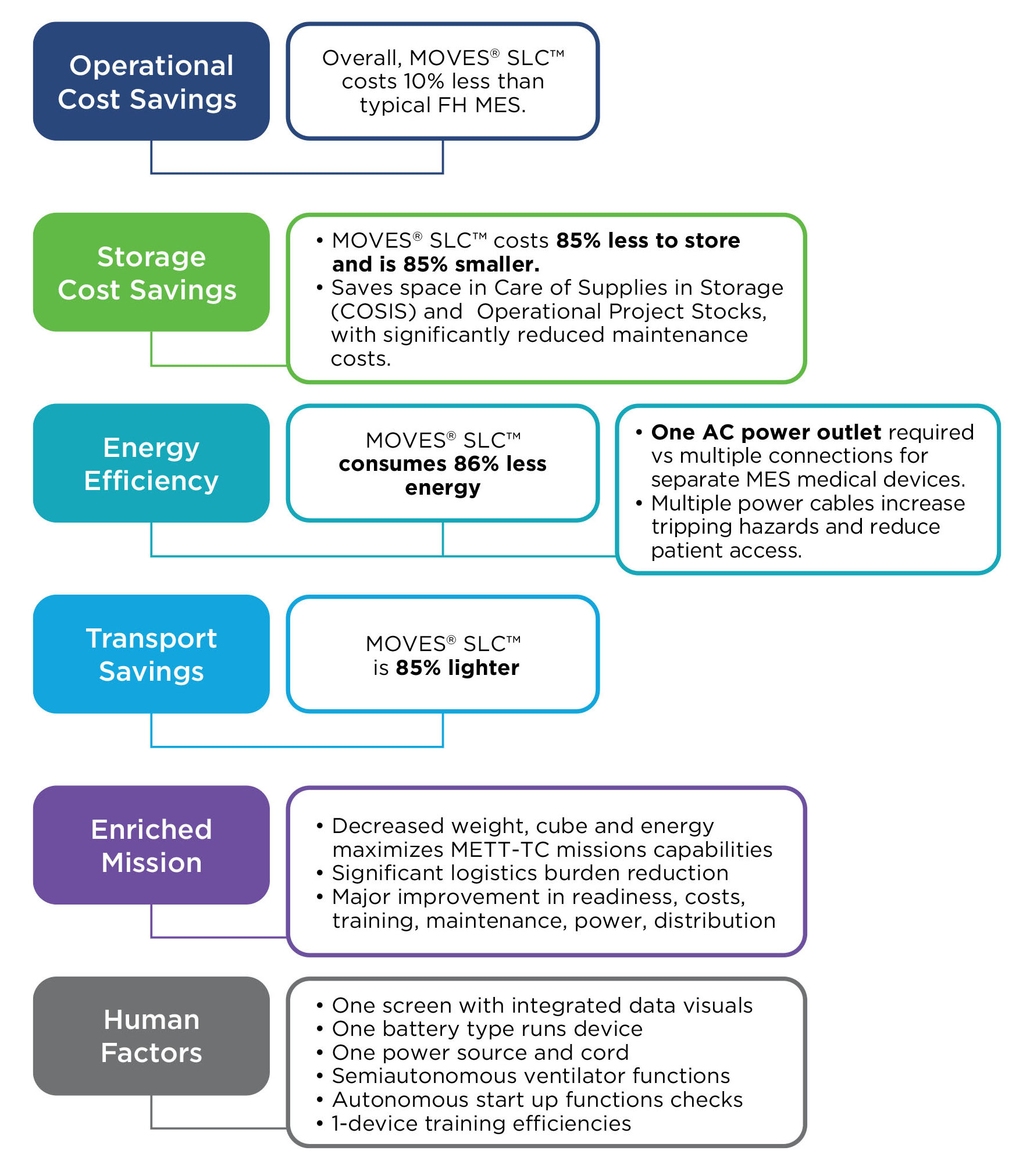
MOVES® SLC™ is ready for these challenges - today.
MOVES® SLC™ is a micro-integrated, mission-ready, portable life support system for complex domains, to modernize casualty damage control, prolonged casualty care and evacuation.



MOVES® SLC™. Agile. Mobile. Self-sufficient.
*Source: Third-party, independent analysis by Capital i, LLC, in 2022, at the request of Thornhill Medical. Results are intended to be guiding only, based on the assumptions presented and the information available at the time of completion, subject to change.
1. Patient Movement = en route medical care and emergency medical intervention; JP 4-02 Ch 1/FM 4-02
2. 100% operational, 10-year lifespan, 24 patients per year, 83% @ Fi02 = 93%; 17% @ Fi02 = 100%
Results are intended to be guiding only, based on the assumptions presented and the information available at the time of completion, subject to change.
Examples of MOVES® SLC™ in Action Since 2017
As presented on YouTube, “Combat medics. Death will wait” tells the story of the unique military medical unit of the Ukraine Special Operations Forces “Alpha” (SOF “A”), where the surgeons of the special SSU unit heroically save lives of the Ukrainian defense fighters every day.
Graphic content included.

Chapter 8: Life or death in Iraq: working in a special operations surgical team.
“To remain mobile, the equipment must of course be as light as possible and compact as possible … It must also all be easy to transport. The equipment must be able to be set up and taken down quickly ... A nice example of these is the MOVES®. A device that produces oxygen, breathes, performs monitoring, has suction and on which accessories can still be mounted. An all-in-one product, such as a smartphone, that can handle multitasking. Thanks to this device we can use a whole range of other devices.”
Geneesheer luitenant-kolonel (Physician Lieutenant Colonel) Bart Vanderheyden

Deployed on October 14, 2023, the ERCS allows the U.S. Navy to evacuate critically ill patients while maintaining full medical capabilities back on the ship. As a component within the ERCS, MOVES® SLC™ enables expanded care capabilities advanced technology that is smaller, lighter, more durable, flexible, easy to maintain, and less reliant on supply chain.
From left, a manufacturer representative provides familiarization training of a mobile, integrated intensive care unit equipment to Lt. Kyle Rowland, a critical care nurse assigned to Navy Medicine Readiness and Training Command (NMRTC) Camp Lejeune, and Hospital Corpsman 2nd Class Bradley Christian, a search and rescue technician assigned to NMRTC Patuxent River, as Cmdr. David Goodrich, the lead research and development of naval expeditionary medical platforms assigned to U.S. Navy Bureau of Medicine and Surgery, observes at Naval Medical Readiness Logistics Command. Rowland and Christian are a part of a 2-person team with the En-Route Care System (ERCS), one of the Navy’s newest medical capabilities that will deploy for the first time with the Eisenhower Carrier Strike Group in order to meet the demands of maritime operations in a distributed environment. U.S. Navy photo by Julius L. Evans. This photo has been altered for security purposes by blurring out access badges. The appearance of U.S. Department of Defense (DoD) visual information does not imply or constitute DoD endorsement.
Modernization to MOVES® SLC™ builds on the first-generation MOVES®, developed in partnership with the USMC, and adopted by the USMC as their Portable Patient Transport Life Support System (PPTLSS) in 2014.
“When you're downrange and a life is on the line, it feels like everything is against you … the smallest thing can make a difference on determining whether someone lives or dies.”
Michael Nace, a former Navy corpsmen and class instructor.

TESTING ORGANIZATIONS

TESTING CERTIFICATIONS